
NASDAQ: VRTX" target="_blank" rel="noopener">Gene therapy will get its share of the spotlight in the medical sector in 2024. The FDA approval for Casgevy, Vertex Pharmaceuticals Inc. (NASDAQ: VRTX) and CRISPR Therapeutics AG (NASDAQ: CRSP) CRISPR/Cas9-based treatment for sickle cell disease (SCD) in December 2023 kicked off the NASDAQ: CRSP" target="_blank" rel="noopener">gene-editing revolution and mainstream attention.
Most gene-editing companies have very little, if any, revenue as they are still building out the technology. However, gene therapy treatments cost the Here are two more gene-editing companies for speculators to keep on their 2024 watchlist.
Beam Therapeutics Inc.
Beam Therapeutics Inc. (NASDAQ: BEAM) develops precision genetic medicines to treat patients suffering from rare genetic diseases using the CRISPR-Cas system.
Base editing is a more precise form of gene editing than CRSPR/Cas9 because it looks to make single-letter (one DNA base pair) changes in DNA, thereby minimizing the chances of unintended consequences. CRISPR/Cas9 introduces double-strand breaks in the DNA to perform deletions, insertions or substitutions. The repair process for Cas9 can incorporate errors and have potential unintended consequences.
Lead drugs
The company's BEAM-101 and BEAM-201 therapies treat sickle-cell disease (SCD) and beta-thalassemia (BT). BEAM-101 is a late-stage ex vivo gene therapy candidate whereby cells are removed from the body, edited, and then infused back into the blood cells using a process of electroporation. It activates fetal hemoglobin production by cutting out the sequence that prevents production. It competes with CRISPR Therapeutics, which has already received FDA approval. It could take a few years to get approved as it's still in Phase 1/2 BEACON clinical trials.
BEAM-201 is a treatment for T-cell acute lymphoblastic leukemia. This in vivo treatment occurs inside the body versus ex vivo, which is outside. It uses multi-base editing by silencing multiple genes to create powerful donor-derived CAR-T cells to help fight the disease and is in Phase 2 clinical trials. BEAM-301 is for the treatment of glycogen storage disease (GSD). BEAM-302 is a treatment for alpha-1 antitrypsin deficiency (AATD).
Check out the sector heatmap on MarketBeat.
Earnings report
Beam Therapeutics reported an EPS loss of $1.22 in its third-quarter 2023 earnings report, beating consensus analyst estimates by 11 cents. Revenues grew 8.9% to $17.2 million versus $17.09 million analyst estimates. The company is still losing around $400 million annually as it continues to push through clinical trials for its therapies.
Clinical trial updates
On January 8, 2024, Beam provided an update on its BEACON Phase 1/2 trials of BEAM-101 treating patients with severe SCD. The first clinical data readout should occur in the second half of 2024.
The European Clinical Trial Application (CTA) was submitted for BEAM-302, with the Phase 1 trial expected to commence in the first half of 2024. The company has over $1 billion in cash, giving it a runway to support operations into 2027.
Beam Therapeutics analyst ratings and price targets are at MarketBeat. Look for Beam Therapeutics peers and competitor stocks with the MarketBeat stock screener.
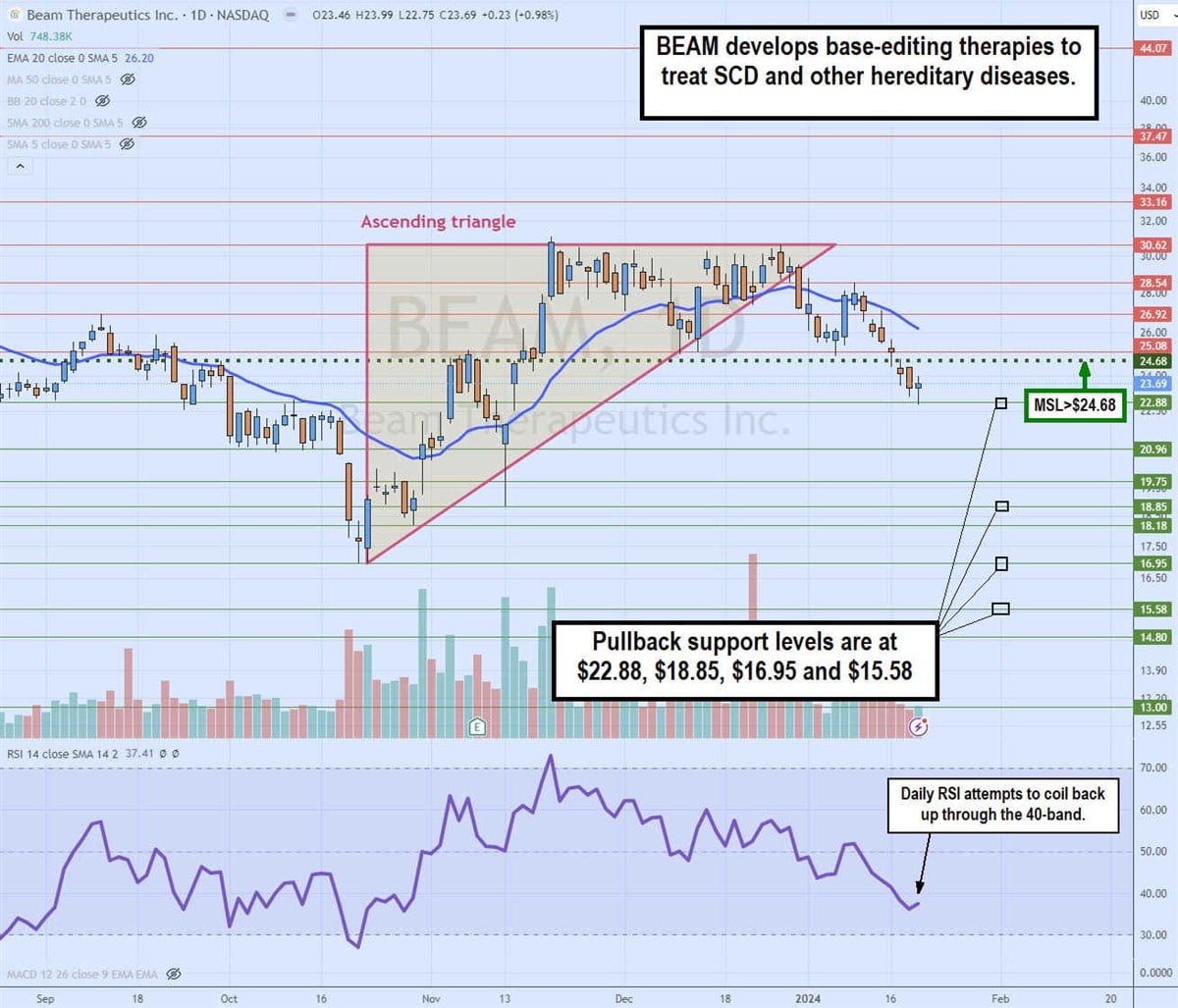
Daily ascending triangle breakdown
The daily candlestick chart for BEAM illustrates a daily ascending triangle pattern. The ascending (rising) trendline commenced at the $16.95 swing low on October 23, 2023. Pullbacks made higher lows up against the flat-top upper trendline at $30.62.
The breakdown occurred on December 29, 2023, as the share fell through the daily 20-period exponential moving average (EMA) at $28.21. Shares fell towards the daily market structure low (MSL) trigger at $24.69 before coiling back up to retest the daily 20-period EMA on January 9. BEAM eventually rejected and sold off for the next six days, hitting a low of $22.88. The daily relative strength index (RSI) has fallen under the 40-band but may be attempting to bounce. Pullback support levels are at $22.88, $18.85, $16.95 and $15.58.
Prime Medicine Inc.
Prime Medicine Inc. (NYSE: PRME) pioneered prime editors to apply prime editing, the next generation of gene editing. Prime editing works to locate the precise place in the human genome to edit and replace the faulty DNA segment with a correct copy — a modified CRISPR/Cas9 enzyme and a prime editing guide RNA (pegRNA) molecule.
The prime editor functions like a word processor to search and replace, enabling single-letter changes within genes without cutting or pasting DNA, causing double-strand DNA breakage and minimizing the potential for off-target effects.
Pipeline
Prime Medicine has 18 early-stage programs focusing on genetic diseases targeting tissues from the blood, liver, eye, ear, lungs and more. Most of the programs are in early discovery and lead optimization stages. Its chronic granulomatous disease (CGD) has reached the investigational new drug (IND) enabling stage. In the IND-enabling stage, a regulatory application is submitted to the FDA to initiate clinical trials of a new drug. Preclinical research, including lab and animal studies, reaches the IND-enabling stage.
Toxicology studies, pharmacokinetics/pharmacodynamics (PK/PD) studies, regulatory strategy and preclinical data are all gathered for submission.
Developments
On November 6, 2023, Prime provided an update regarding completing its first IND filing in 2024. The company lost 55 cents per share and generated no revenues for its Q3 2023.
The company has initiated IND-enabling studies for PM359 in CGD. It expanded preclinical proof-of-concept data in vivo and in vitro across many programs and tissue types. It continues to optimize viral and non-viral delivery systems to illustrate excellent off-target profiles for its Prime Editor. Its first IND filing will be in 2024, with additional filings in 2025. Prime filed for a $500 million mixed-shelf offering.
On January 5, Prime resolved all outstanding disputes with Myeloid Therapeutics, ending the pending arbitrations and a positive outcome for both parties.
Analyst downgrade
On January 16, Stifel downgraded Prime Medicine stock from a "hold" to a "buy" and lowered its price target to $9, down from $18. The firm noted that 2024 presents no major catalysts as data readouts aren't due until 2025. It noted that shares will likely be rangebound with better entry points later in the year.
Get AI-powered insights on MarketBeat.
Check out Prime Medicine analyst ratings and price targets, and peers and competitor stocks with the MarketBeat stock screener.
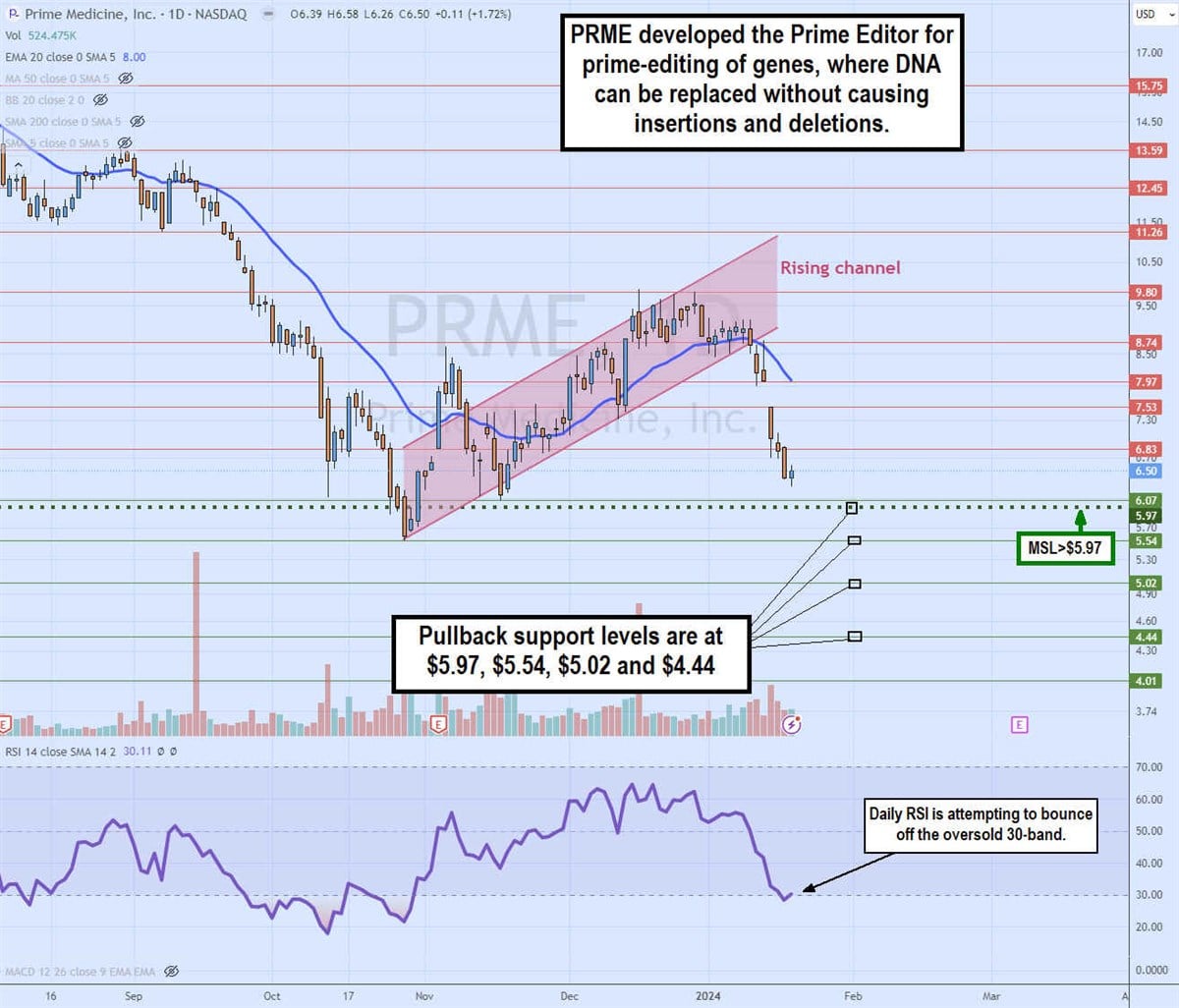
Daily rising channel breakdown
The daily candlestick chart on PRME illustrates a rising price channel. The channel formed after hitting a low of $5.54 on October 27. PRME staged a rally after triggering the daily market structure low (MSL) breakout through $5.97.
The daily 20-period exponential moving average (EMA) held support, rising as shares reached $9.80 in December 2023. However, PRME crumbled through the daily 20-period EMA, falling to $8 in January 2024 as shares attempted to hold above the daily MSL trigger level. The daily relative strength index (RSI) is trying to bounce off the oversold 30-band.




