Hot stocks today: Two companies involved in developing treatments for the Ebola virus saw high-volume activity in the stock market today.
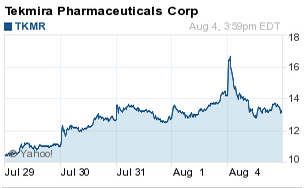
Shortly after today's opening bell, Tekmira Pharmaceutical (Nasdaq: TKMR) stock surged more than 12% to $16.87 on brisk volume. That was on top of an 11% gain on Friday. By 2 p.m., 9 million shares had changed hands, marking the stock's busiest day in its 14-year history. The stock's average daily volume is 472,915 shares.
TKMR stock quickly fell back to earth after a CNN report suggested a rival company will be the first to see its Ebola treatment used widely in the fight against the worst outbreak of the disease in West Africa's history. Tekmira stock not only shed all of today's gains, the stock was in the red by 7% at $13.26.
Tekmira and Competitors Race for Ebola TreatmentA handful of pharmaceutical companies are working with the U.S. military to help find a drug that can take on the deadly virus, which has killed more than 700 people and infected more than 1,000 since March in Guinea, Sierra Leone, and Liberia.
The Ebola virus kills roughly 60% of people infected. Transmitted through human or animal contact, it seems to emerge without notice. Only a few facilities are equipped to handle the virus, which has no cure.
Tekmira has been working on an Ebola treatment for four years. Tekmira has a $140 million contract with the U.S. Department of Defense's Medical Countermeasure Systems BioDefense Therapeutics Joint Product Management Office to develop its drug TKM-Ebola.
The drug uses "Lipid Nanoparticle" technology, which sends the drugs through the patient's bloodstream with tiny oil particles.
The process, called small interfering RNA, has saved primates injected with a fatal amount of the virus. Researchers dosed their first human subjects in a clinical trial in January, but the drug is nowhere ready for widespread distribution. The clinical study for TKM-Ebola was suspended in July by the U.S. Food and Drug Administration amid safety concerns.
Still, Tekmira's drug did receive Fast Track designation in March. That means that "frequent communication between the FDA and a drug company is encouraged throughout the entire drug development and review process" and that the process "assures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients."
Tekmira (Nasdaq: TKMR) Stock Takes a HitBut TKMR stock swooned after CNN reported two ailing American missionary workers suffering with Ebola were treated, with some success, by a drug from a competing biotech company: Mapp Biopharmaceuticals.
Mapp, founded in 2003 and based in San Diego, Calif., is a privately held company concentrating on developing novel pharmaceuticals for the prevention and treatment of infectious diseases. Its key focus is on unmet needs in global health and biodefense.
Researchers at Mapp aim to fight Ebola using a drug developed with tobacco leaves.
One method involves a two-step process. The first step is passive immunization, which means the antibodies attack the virus directly. The second step is active immunization, something that helps boost patients' immune system to fight back on its own accord.
Another Mapp team has uncovered a way to combine these treatments using plants as a pharmaceutical production line.
"We can manufacture both of these post-Ebola exposure reagents for a defensive stockpile, using tobacco," said Charles Arntzen, a researcher at Arizona State's Biodesign Institute, who authored a paper on the subject in an Arizona State University report.
Tests conducted on mice were broadly successfully, with an 80% survival rate. The technology, however, is still far away from being tested on humans.
Tekmira Still in the RaceJason Kolbert, a Maxim Group analyst who covers Tekmira, told CNN it wasn't a surprise to hear that Mapp's serum was used on the two ailing American missionaries since several companies are working with the government on an Ebola therapy.
Kolbert added that just because another medication was used, it doesn't mean Tekmira's drug won't ultimately emerge as a viable Ebola treatment.
Others agree.
"We have solid pre-clinical evidence showing that TKM-Ebola is effective at eradicating Ebola symptoms, giving us confidence that its development activities could resume," Euro Pacific Canada analyst Douglas Loe told Reuters.
Kolbert rates Tekmira a "Buy." Investors, however, should proceed with caution. Like the majority of small biotechs, Tekmira has yet to turn a profit and revenue is small at best.
For better investments in biotech today, go here to read our guide to investing in the bioscience sector in 2014.
Related Articles:
- CNN Money: Ebola Drugmaker's Stock Surges
The post Tekmira Pharma (Nasdaq: TKMR) Stock Falls in Race for Ebola Treatment Approval appeared first on Money Morning - Only the News You Can Profit From.